Radical Resection Of A Thymic Tumor: A Case Report
Christopher M Bobba MD, PhD1*, James Wymer MD2, Olusola Oduntan MD1, Mindaugas Rackauskas MD1
1 Division of Thoracic and Cardiovascular Surgery, The University of Florida, USA.
2 Division of Neuromuscular Neurology, The University of Florida, USA.
*Corresponding Author
Christopher M Bobba,
Division of Thoracic and Cardiovascular Surgery, The University of Florida, USA.
Email: christopher.bobba@surgery.ufl.edu
Received: May 02, 2022; Accepted: May 23, 2022; Published: May 25, 2022
Citation: Christopher M Bobba MD, PhD, James Wymer MD, Olusola Oduntan MD, Mindaugas Rackauskas MD. Radical Resection Of A Thymic Tumor: A Case Report. Int J Surg
Res. 2022;8(4):165-166.
Copyright: Christopher M Bobba© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Thymic carcinoma can demonstrate rapid local growth and invade surrounding mediastinal structures. Surgery
remains the mainstay of therapy, provided the tumor can be completely resected.
Case Presentation: A 71-year-old female with worsening myasthenia gravis was found to have a large thymic tumor. Chest
CT scan indicated possible vascular and pleural invasion. We performed surgery combining vascular reconstruction of the innominate
vein, pericardial patch replacement, and bilateral upper lobe wedge resections to achieve complete local excision of
this tumor.
Conclusion: Resection of thymic carcinoma can require a wide breadth of surgical technique, including vascular reconstruction,
and meticulous mediastinal and pleural dissection.
2.Case Report
3.Discussion
4.References
Introduction
Thymic cancer is a rare cancer involving the thymus in the anterior
mediastinum, andsurvival is dependent upon histologic subtype.
Thymic carcinoma is the most aggressive, and readily invades
adjacent structures.[1] They can also present with paraneoplastic
myasthenia gravis. Surgical resection remains the mainstay of
therapy.Here we report on a patient with progressing myasthenia
gravis who had athymic tumor requiring resection and graft replacement
of innominate vein, pericardial patch reconstruction
and bilateral upper lobe wedge resection.
Case Report
A 71-year-old female with arthritis and spinal stenosis presented
to a neurologist with new onset proptosis and anxiety and was
started on pyridostigmine. A chest computerized tomographic
(CT) scan was scheduled for several weeks later. In the interim,
she developed progressive dysphagia, shortness of breath, and
worsening proptosis. She was admitted to the hospital and treated
with intravenous pyridostigmine. Chest CT scan obtained while
inpatient demonstrated a large mediastinal soft tissue mass with
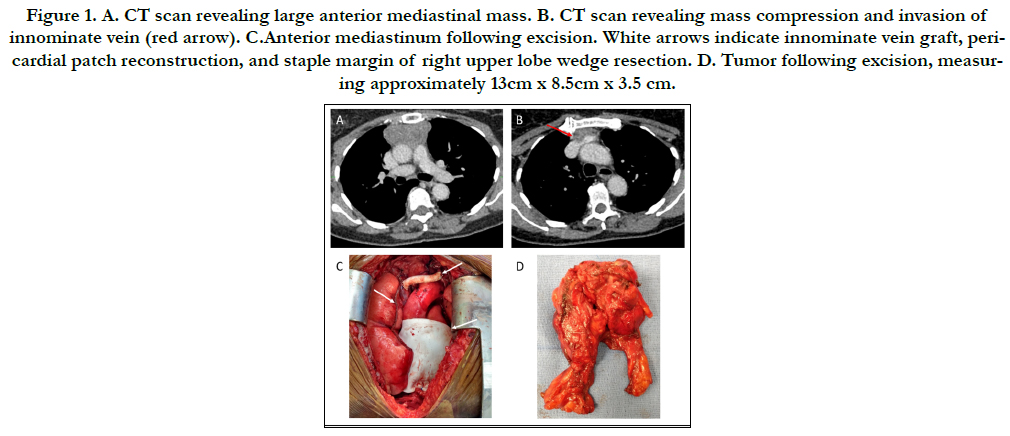
internal calcifications (Figure 1A) and possible vasculature invasion
(Figure 1B). The patient initially declined medical or surgical
treatment. She presented to our thoracic surgery clinic one month
later with worsening myasthenic symptomsand elected to proceed
with surgery at that time. Prior to surgery she underwentplasma
exchange.
.
A median sternotomy was performed. The mass was apparent in
the anterior mediastinum, with adherence to the right and left upper
lobes. Bilateral upper lobe wedge resectionswere performed
in contiguity with the tumor. Dissection identified invasion into
the left innominate vein and pericardium. Systemic heparin was
administered, and vascular control of the vein was achieved.
5.5cm of the left innominate vein was resected, extending to the
junction with the superior vena cava. An 8mm vascular graft was
interposed for reconstruction. The pericardium was resected to
the margin of the phrenic nerves including removal of adventitia
from ascending aorta and aortic arch. Pericardium was reconstructed
with a Gore-tex patch (Figure 1C). Pathology identified
the tumor as thymic carcinoma (Figure 1D). Surgical margins of
innominate vein, superior vena cava, and lung sections were negative.
Eight lymph nodes were negative.
She recovered well. Three chest tubes were placed at the conclusion
of the case, and were removed sequentially. The patient had
near complete resolution of myasthenic symptoms and was reinitiated
on pyridostigmine. She was discharged on post-operative
day six in excellent condition. At her 9-month follow-up, she continued to do very well. She had completeresolution of myasthenic
symptoms and was tapered off pyridostigmine. There were no
signs of recurrent disease on CT scan and she had completed a
3-month course of dual antiplatelet therapy.
Figure 1. A. CT scan revealing large anterior mediastinal mass. B. CT scan revealing mass compression and invasion of innominate vein (red arrow). C.Anterior mediastinum following excision. White arrows indicate innominate vein graft, pericardial patch reconstruction, and staple margin of right upper lobe wedge resection. D. Tumor following excision, measuring approximately 13cm x 8.5cm x 3.5 cm.
Discussion
Our patient had Masaoka Stage IIIa, WHO Type C, and AJCC
Stage IIIA (T3N0M0) thymic carcinoma.Following National
Comprehensive Cancer Network (NCCN) guidelines for an R1
resection (microscopic evidence of residual tumor in the pericardium),
we recommended adjuvant radiation therapy ± chemotherapy,
and medical oncologist consultation. Post-operatively our
patient had improved myasthenic symptoms and was uninterested
in any additional therapy, despite extensive counseling about recurrence.
The standard of care for locally advanced non-metastatic thymic
tumors remains surgical resection whenever feasible ± neoadjuvant
chemoradiation. The complexity of the case is largely
determinant on invasion of surrounding structures and tumor
size. Individual reports have demonstrated successful resection
of thymic carcinomas invading local vascular [2] or pleural invasion.[
3] Invasion of the superior vena cava or innominate vein
may necessitate graft replacement. Invasion of other great vessels
may also occur. These cases require meticulous dissection of the
tumor and the awareness that graft replacement may be necessary.
In addition to vascular invasion, tumor may also spread into
the pericardium, pleura and lungs. The surgeon must be aware
of these possibilities and the need for possible pericardial reconstruction
or lung resection. In this case, tumor invasion into the
right lung, innominate vein and pericardium required use of all
these eventualities. Following vascular graft placement,we opted
for three months of anticoagulation therapy to ensure adequate
time for graft epithelialization, though little data exist to guide
antiplatelet therapy in venous vascular grafting. The patient completed
this course of therapy without consequence. Given the
central anatomic location of the thymus within the mediastinum,
thymic tumor resections are often challenging and unique cases.
This report highlights the complexity and wide breadth of surgical
skill necessary for the surgical resection of advanced thymic
tumors.
References
- Shintani Y, Funaki S, Ose N, Kanou T, Fukui E, Minami M. Surgical approach for thymic epithelial tumor. J Thorac Dis. 2019 Aug;11(8):E127- E130.
- Momozane T, Inoue M, Shintani Y, Funaki S, Kawamura T, Minami M, et al. Trimodality Therapy for an Advanced Thymic Carcinoma With Both Aorta and Vena Cava Invasion. Ann Thorac Surg. 2016 Aug;102(2):e139-41. Pubmed PMID: 27449450.
- Shintani Y, Kanzaki R, Kusumoto H, Nakagiri T, Inoue M, Okumura M. Pleuropneumonectomy for a large thymoma with multiple pleural dissemination using median sternotomy followed by posterolateral thoracotomy. Surg Case Rep. 2015;1(1):75.Pubmed PMID: 26366371.