Effect of Plant Extracts on Innate Immunity of Skin Cells: Investigations on Human Keratinocyte Cell Lines
Samiah Hamad Al-Mijalli1*, Barbara Plancot2
1 Biology Department, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
2 Université de Rouen, Scientific Section, 76 821 Mont saint Aignan, France.
*Corresponding Author
Dr. Samiah Hamad Al-Mijalli,
Biology Department, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Tel: 00966555200510
Fax: 009664871229
Email: dr.samiah10@hotmail.com
Recieved: April 23, 2020; Accepted: June 29, 2020; Published: July 27, 2020
Citation: Samiah Hamad Al-Mijalli, Barbara Plancot. Effect of Plant Extracts on Innate Immunity of Skin Cells: Investigations on Human Keratinocyte Cell Lines. Int J Microbiol Adv Immunol. 2020;7(1):96-105.doi: http://dx.doi.org/10.19070/2329-9967-2000019
Copyright: Samiah Hamad Al-Mijalli© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Stresses caused by many factors including pathogenic bacteria result in inflamatory diseases and they are considered as a major
source of skin cancers and premature ageing.
Plants growing in semi-arid environments and deserts present the astonishing capacity to survive extreme conditions of
drought and high temperatures. The objective of this study is to investigate the effects of extracts from two endemic plants
from Saudi Arabia, namly C. myrrha and Rhamnus frangula, on the immune response of human keratinocyte cell lines (Ha-
CaT).
Our major findings are i) the extracts from both plants exhibit a significant anti-oxidant activity. The concentrations used are
below the limit value of toxicity. ii) These extracts stimulate the innate immune response in HaCaT cells and iii) treatments
with these extracts are not harmful to the natural cutaneous microflora.
Together these findings are highly promising with regards to skin protection.
It was in this work investigate the impact of plant extracts on the immune response of human keratinocyte cell lines (HaCaT).
In this study, the elicitor activity of the extracts obtained from two endemic plants Arabia C. myrrha and R. frangula was evaluated.
These plants were collected and provided by Dr. Samiah Hamad Al-Mijalli from (Saudia Arabia). The antioxidant activity
of C. myrrha and R. frangula extracts was assessed for its ability to scavenge free radicals and to protect human keratinocytes
from oxidative stress. Furthermore, the impact of such treatment was evaluated on both HaCaT cell viability and on the
growth/proliferation of the natural cutaneaous microbiota.
2.Abbreviations
3.Introduction
4.Material and Methods
5.Results
6.Discussion
7.Acknowledgement
8.References
Keywords
Bacteria; Commiphora Myrrha; Immunity; Rhamnus Frangula; Skin.
Abbreviations
A Chromophore Termed Bathocuproine (BATO); Commiphora myrrha (C. myrrha); Dulbecco’s modified
Eagle’s medium (DMEM); Human keratinocyte cell lines (HaCaT); Oxygen Radical Absorbance Capacity (ORAC); Potent
oxidant, 2,2_- azobis (2-amidinopropane) dihydrochlorid (AAPH); Rhamnus frangula (R. frangula); Reactive oxygen species
(ROS); Total Anti-oxidant Capacity (TAC).
Introduction
The skin is a natural barrier between the body and the environment
and is colonized by a large number of microorganisms.
Environmental aggressions such as UV exposure, tobacco and
pathogenic micro-organisms are known to have deleterious effects
on skin ultrastructure thus resulting in a loss of its protective
function. Chronic or excessive environmental stresses lead
to persistent inflamatory disease considered as a major source of
skin cancers and premature ageing [4]. As a result of these various
aggressions, the skin is constantly exposed to reactive oxygen species
(ROS) production causing the so-called oxidative stress. ROS are highly toxic to the skin layers, and ROS-mediated oxidative
damage involves many biological molecules such as DNA modification
(e.g., changes in DNA methylation), lipid peroxidation and
secretion of inflammatory cytokines [7].
It has long been recognized that many naturally-occurring substances
in plants have antioxidant activities [2]. Multipotent antioxidants
are molecules that in addition to antioxidant activity
possess pharmacological activitues. There are numerous examples
of multipotent antioxidants among natural products. Besides
their antioxidant properties, some natural antioxidants inhibit
platelet-aggregation or display antineoplastic, anti-inflammatory
and other activities [3]. The antioxidant activity of plant extracts is
of particular interest both because of their beneficial physiological
activity on human cells and the potential they have to replace
synthetic antioxidants [6].
The objective of the present study is to take advantages of two
endemic plants of Saudi Arabia and native to desert area. Such
plants are known to be adapted to extreme environmental conditions
and develop fascinating strategies to cope with both drought
stress and extreme temperature. As a consequence, we postulated
that these unexplored plants might be a source of new natural
products that can potentially be used in cosmetic and dermatology.
HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM, Gibco®) upplemented with 10% fetal bovin serum
(FBS south american, Gibco®) and 1% penicillin–streptomycin
at 37°C in a 5% CO2 humidified atmosphere.
Briefly, the skin specimens were washed with DMEM, trypsinized
(0.25% trypsin) at 37°C for 3min to detach. Cells were collected
and placed onto 96. Keratinocytes were used for experiments at
60–80% of confluence.
Before all measurements/experiments, HaCaT cells were starved
in DMEM without addition of FBS for 24 h. The starvation conditions
were necessary to uniform the experiments and exclude
any undesired interference of serum components with cell response
to the stimulus.
In situ detection of general ROS was performed by staining Ha-
CaT cells cells with the probe CM-H2DCFDA , which is nonfluorescent
in its reduced form. In living cells, cell esterases convert
CM-H2DCFDA into 2,7-dichlorodihydrofluorescein diacetate,
which is subsequently oxidized by ROS into the fluorescent form
2,7-dichlorofluorescein in the presence of ROS. 2 104, maintained
in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS
under 5% CO2 at 37°C, were seeded in 96-well plates and grown
for 24 h to let them attach to the bottom of the well. The cells
were then incubated with different concentrations of hot water
plant extracts at concentrations ranging from 0.031 mg.mL-1 to
4 mg.mL-1 to R. frangula and C. myrrha for 45 min, and then incubated with 100μl L of 5 μM CM-H2DCFDA. The fluorescence
of the samples was thus measured at 535nm (excitation 490 nm),
using the instrument (Flexstation 3, PRIMACEN), and from each
measure the cell auto-fluorescence value was subtracted.
Effects of stress exposures and their combination with hot water
plants extracts on HaCaT cells growth and vitality as well as
membrane integrity were assessed after 24 h by standard assays.
The keratinocyte proliferation and cell survival was determined by
MTT (3-[4,5-dimethylthiazol-2yl]-diphenyl tetrazolium bromide)
colorimetric assay described elsewhere. The vitality and growth
rate determinations were performed in triplicate for each experimental
condition.
Both the assays were used to measure the total anti-oxidant power
of the hot water plant extracts by “in vitro” reactions. The Total
Anti-oxidant Capacity (TAC) assay is based on a redox reaction
between a test compound and copper II, Cu (II). If a test compound
has got reducing power, the Cu (II) is readily converted
into Cu (I) which can be monitored by the addition of a chromophore
termed Bathocuproine (BATO). Fifty μl of hot water plant
extracts solution in PBS (at concentrations ranging from 0.031
mg.mL-1 to 4 mg.mL-1 to R. frangula and C. myrrha ) was aliquoted
into a 96-well plate. 50μl of a BATO solution (360μM)
was added to each well and the background absorbance at 490nm
was measured, then 25μl of 100μM CuSO4 solution was added
to each sample and incubated at room temperature for 30 min.
At the end of the incubation time, the absorbance at 490 nm was
measured by a plate reader (Victor3, PerkinElmer). As reference
standard, scalar dilutions of CuCl were used, ranging from 30 to
10 mM.
Oxygen Radical Absorbance Capacity (ORAC) assay is based on
the ability of a test compound to inhibit the oxidation of a fluorophore,
generally fluorescein, by a potent oxidant, 2,2_- azobis
(2-amidinopropane) dihydrochloride (AAPH). Twenty-five μl of
hot water plant extracts dilutions in phosphate buffer 75mM, pH
7.4 (at concentrations ranging from 0.031 mg.mL-1 to 4 mg.mL-1
to R. frangula and C. myrrha) was aliquoted into 96-well plate and
150μl of fluorescein solution (2.5 nM in phosphate buffer) was
added to each sample. After incubation at 37 °C for 15 min, 25μl
of AAPH solution (153mM in phosphate buffer) was pipetted
into each well and the progress of the reaction monitored at 535
nm, using a fluorescence multi-well reader. The fluorescence was
measured every minute for 5 min.
2 104 HaCaT cells are maintained in Dulbecco’s modified Eagle’s
medium (DMEM) with 10% FBS under 5% CO2 at 37°C, were
seeded in 96-well plates and grown for 20 h to let them attach
to the bottom of the well. The cells were then incubated with
different concentrations of hot water plant extracts or ascorbate
250μM, used as positive control, for 2 h. At the end, the cells
were washed in PBS and the cell auto-fluorescence value (background)
measured at the plate reader, using 490nm as excitation
and 535nm as emission wavelength. The cells were then incubated with the dye CM-H2DCFDA (5-(and-6)-chloromethyl-2,7-
dichlorodihydrofluorescein diacetate, Invitrogen) at 37°C for 30
min. After an additional wash in PBS, the cells were treated with
H2O2 150μM and incubated for 30 min. The fluorescence of the
samples was thus measured at 535nm (excitation 490 nm), using
the instrument (Flexstation 3, PRIMACEN), and from each
measure the cell auto-fluorescence value was subtracted.
Total RNA was extracted using an RNeasy mini kit (Qiagen)
in accordance with the manufacturer’s instructions. The RNA
was eluted with 22 μl of RNase-free water. DNase I treatment
(RNase-Free DNase Set; Qiagen) was performed to remove contaminating
genomic DNA. The quality of the RNA was assessed,
and the quantity was estimated by nanodrop.
Approximately 1μg of RNA were reverse-transcribed in a 20 μl
reaction by High Capacity cDNA Reverse Transcription Kits (Applied
biosystem) that contained 10X RT Buffer, 10X RT Random
Primers, 25X dNTP Mix (100 mM), MultiScribe™ Reverse Transcriptase
50 U/μL and RNase Inhibitor.
Quantitative real-time PCR amplification reactions were performed
using a 7500 Fast Realtime PCR System (Applied Biosystems)
in a final reaction volume of 13μl, which contained fast
SYBR Green Master Mix (Applied Biosystems), 300 nM forward
and reverse primers and the template cDNA. The thermal cycling
conditions involved a denaturation step at 95°C for 20 s, followed
by 40 cycles of amplification at 95°C for 3 s and 60°C for 30 s.
The final dissociation step was performed at 95°C for 15 s, 60°C
for 1 min, 95°C for 15 s and 60°C for 15 s.
To determine if treatment with plant extracts induced oxidative
stress in keratenocytes HaCaT, ROS production was monitored
based on different concentrations with the probe CM-H2DCFDA
able to detect all the ROS.
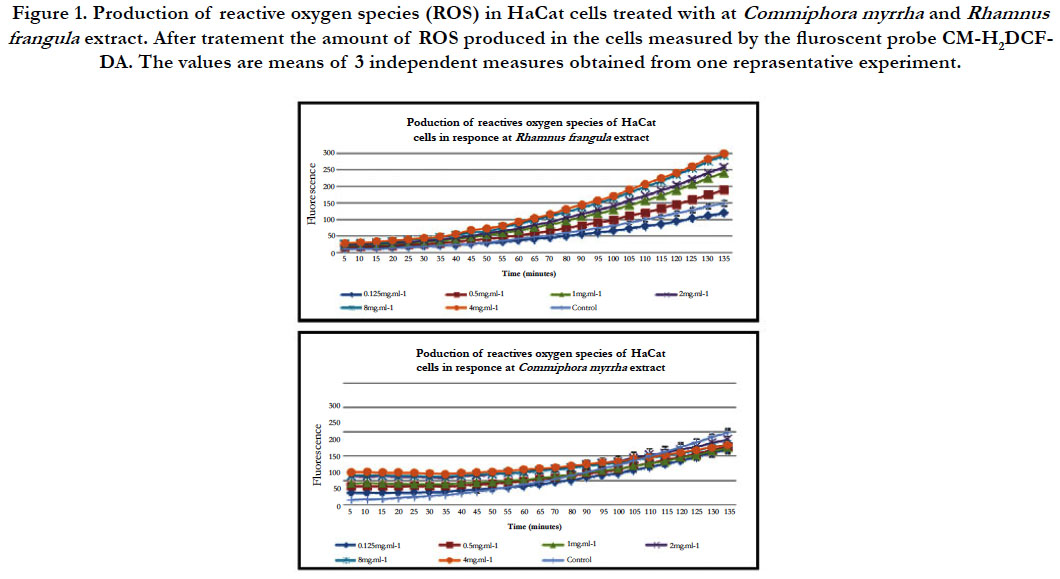
The hot water extract of R. frangula generates ROS in HaCaT cells.
Moreover, production of ROS by HaCaT cells increases with increasing
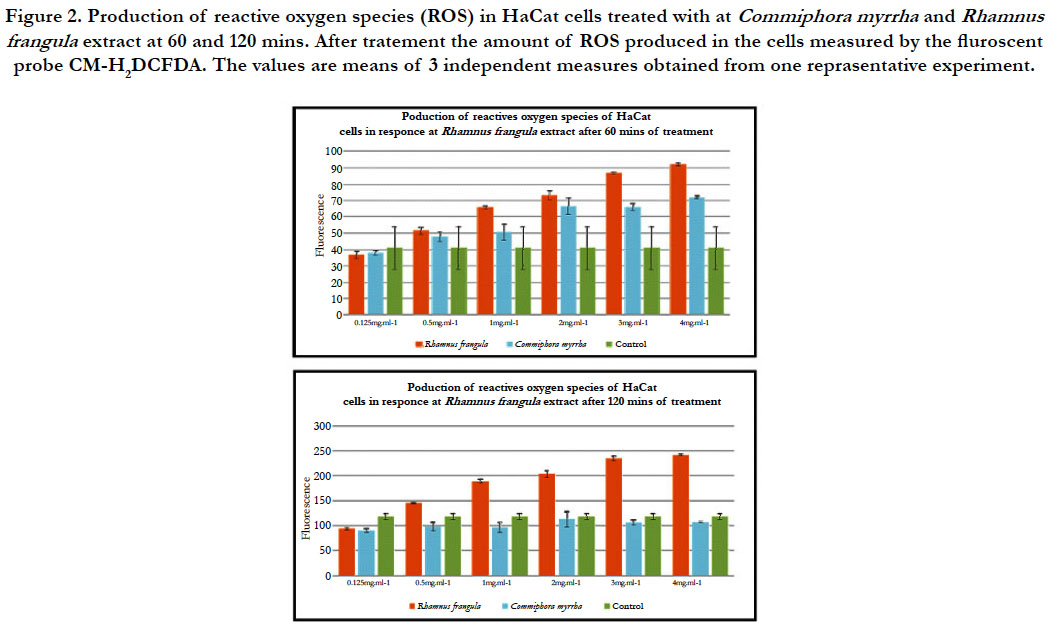
concentrations (figure 1). Indeed, after 60 minutes of
treatment with R. frangula hot water extracts, a significant increase
of ROS production is observed for the following concentrations
0.5 mg.mL-1, 1 mg.mL-1, 2 mg.mL-1, 3 mg.mL-1 et 4 mg.mL-1
compared with HaCaT cells treated with PBS (Phosphate Buffer
saline) buffer only (figure 2). After two hours of treatment, the
generation of ROS continues to increase to reach an equivalent
fluorescence 200 AU, and 2 times higher than the PBS control
(figure 2).
Figure 1. Production of reactive oxygen species (ROS) in HaCat cells treated with at Commiphora myrrha and Rhamnus frangula extract. After tratement the amount of ROS produced in the cells measured by the fluroscent probe CM-H2DCFDA. The values are means of 3 independent measures obtained from one reprasentative experiment.
The hot water extract of C. myrrha gererate ROS in HaCaT cells within the first hour after treatment (figure 1). Moreover, the concentration increases and more production of ROS by HaCaT cells is observed. After 60 minutes of treatment with C. myrrha hot water extracts, a significant increase of ROS production is observed for the concentrations 0.5 mg.mL-1, 1 mg.mL-1, 2 mg.mL-1, 3 mg.mL-1 et 4 mg.mL-1 compared to HaCaT cells treated with PBS (figure 2). However, after sixty minutes, the trend reverses. The hot water extract of C. myrrha does not induce the generation of ROS compared to HaCaT cells treated with the buffer PBS. After 120 min with the treatment PBS, the HaCaT cells produce much more ROS than HaCaT cells treated with the hot water extract of C. myrrha (figure 2).
Figure 2. Production of reactive oxygen species (ROS) in HaCat cells treated with at Commiphora myrrha and Rhamnus frangula extract at 60 and 120 mins. After tratement the amount of ROS produced in the cells measured by the fluroscent probe CM-H2DCFDA. The values are means of 3 independent measures obtained from one reprasentative experiment.
In order to determine if the hot water of different plant extracts
are cytotoxic to HaCaT cells, the cell viability and cell proliferation
were determined by the MTT assay at different concentrations
according to the method of Apone et al., [1].
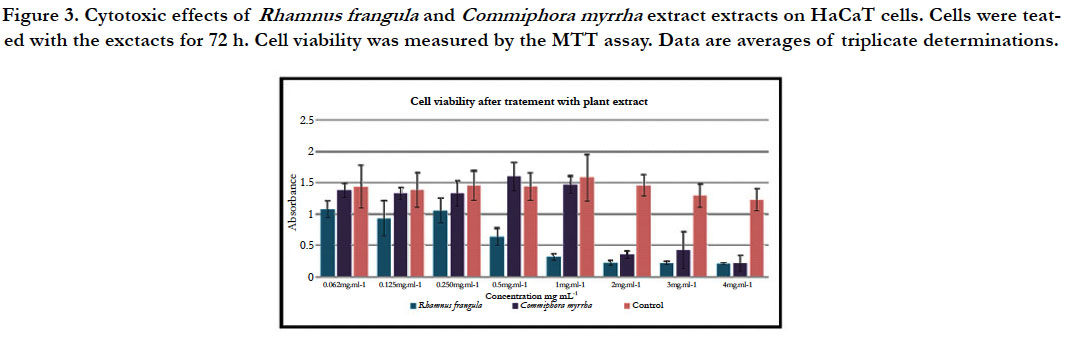
Treatment with the hot water extract of R. frangula reduces the viability
of the cells and proliferation at concentrations higher than
0.5 mg.mL-1. However, concentrations below 0.5 mg.mL-1 had no
significant effect on the viability and proliferation of HaCaT cells.
Treatment with the hot water extract of C. myrrha reduces the viability
and proliferation at concentrations higher than 1 mg.mL-1.
However, concentrations below 1 mg.mL-1 have no significant effect
on the viability and proliferation of HaCaT cells (figure 3).
Figure 3. Cytotoxic effects of Rhamnus frangula and Commiphora myrrha extract extracts on HaCaT cells. Cells were teated with the exctacts for 72 h. Cell viability was measured by the MTT assay. Data are averages of triplicate determinations.
In vitro assays: The anti-oxidant activity of a compound or a
mixture of compounds depends on its total reducing capacity,
on its ability to penetrate into the cells, and on its capacity to
trigger a signal cascade leading to the production of endogenous
anti-oxidant compounds in the cells. “In vitro” assays measure the
reducing potential of the test material; however, they do not provide
any information on the anti-oxidant induction in living cells.
Total Anti-oxidant Capacity (TAC): The Total Anti-oxidant
Capacity (TAC) assay is based on a redox reaction between a test
compound and copper II, Cu (II). If a test compound has a reducing
power, the Cu (II) is readily converted into Cu (I) which
can be monitored by the addition of a chromophore termed
Bathocuproine (BATO).
For the hot water extracts of R. frangula and C. myrrha different
concentrations (ranging from 0.062 mg.mL-1 to 4 mg.mL-1) were
mixed with 100mM of Cu (II) and BATO, and after 30 min the
amount of Cu (I) produced was measured at 490 nm.
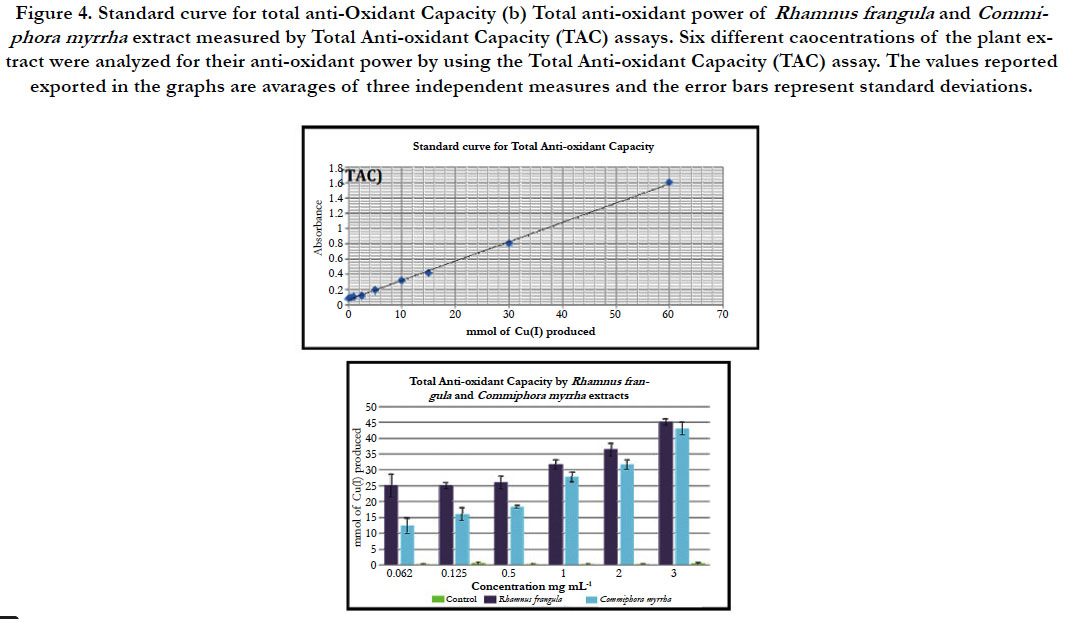
For the concentrations ranging from 0.062 mg.mL-1 to 0.5 mg.mL–1
of the hot water extract of R. frangula, the quantity of mmol of
Cu (I) produced per liter remains constant and is equivalent to 25
mmol. However, with increasing concentrations of R. frangula hot
water extracts, the quantity of Cu (I) product per liter increases to
reach 45 mmol at a concentration of 4 mg.ml-1. In the presence
of the PBS (control), the amount of mmol of Cu (I) produced is
equivalent to zero.
For the concentrations, ranging from 0.062 mg.mL-1 to 4 mg.mL–1
of the hot water extract of C. myrrha, the quantity of mmol of
Cu (I) produced per liter increased in relation with the concentrations
used to reach 42 mmol at a concentration of 4 mg.ml-1. In the presence of the PBS (control), the amount of mmol of Cu (I)
produced is equivalent to zero (figure 4).
Figure 4. Standard curve for total anti-Oxidant Capacity (b) Total anti-oxidant power of Rhamnus frangula and Commiphora myrrha extract measured by Total Anti-oxidant Capacity (TAC) assays. Six different caocentrations of the plant extract were analyzed for their anti-oxidant power by using the Total Anti-oxidant Capacity (TAC) assay. The values reported exported in the graphs are avarages of three independent measures and the error bars represent standard deviations.
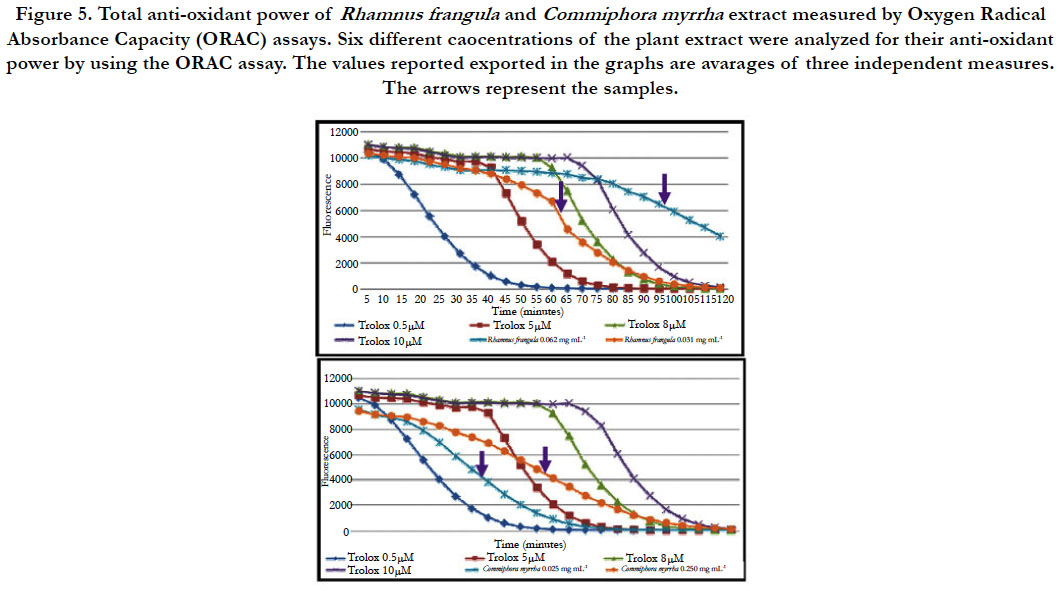
Oxygen Radical Absorbance Capacity (ORAC): Oxygen Radical Absorbance Capacity (ORAC) assay is based on the ability of a test compound to inhibit the oxidation of a fluorophore, generally fluorescein, by a potent oxidant, 2, 2- azobis (2-amidinopropane) dihydrochloride (AAPH). The standard is a powerfull anti-oxidant, the trolox (vitamin E) with the concentration ranging from 0.5 μM to 20 μM.
For the hot water extract of R. frangula at the concentration of 0.031 mg.mL-1, the values are comprised between the curves of the trolox equivalent to 5 μM and 8 μM. Thus, the antioxidant activity of hot water extracts of R. frangula is equivalent to the concentration of trolox 5 - 8μM. At the concentration 0.062 mg.mL-1 the curve is located after the curves of the trolox equivalent to 10μM. Thus, the antioxidant power of hot water extract of R. frangula is equivalent to the concentration of trolox higher at 10μM of trolox (figure 5).
Figure 5. Total anti-oxidant power of Rhamnus frangula and Commiphora myrrha extract measured by Oxygen Radical Absorbance Capacity (ORAC) assays. Six different caocentrations of the plant extract were analyzed for their anti-oxidant power by using the ORAC assay. The values reported exported in the graphs are avarages of three independent measures. The arrows represent the samples.
For the hot water extract of C. myrrha at the concentration of 0.125 mg.mL-1 and 0.250 mg.mL-1, the curve is located between the curves of the trolox equivalent to 0.5 μM and 5 μM. Thus, the antioxidant power of hot water extract of R. frangula is equivalent to the concentration of trolox 0.5-5 μM (figure 5).
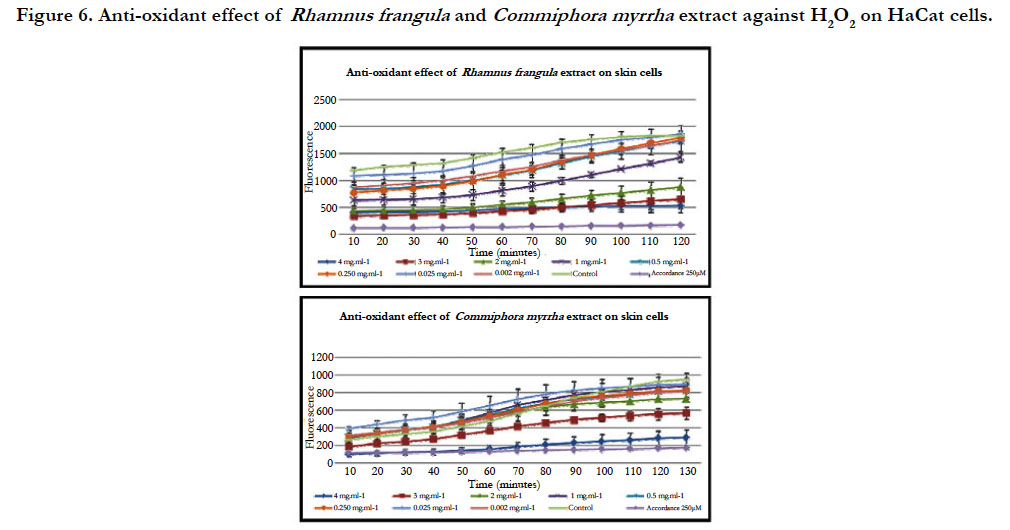
2 In vivo assays: To evaluate the protective anti-oxidant effect of the plant extract on the HaCaT cells, we treated the cells with eight different concentrations of the mixture before being stressed by H2O2.
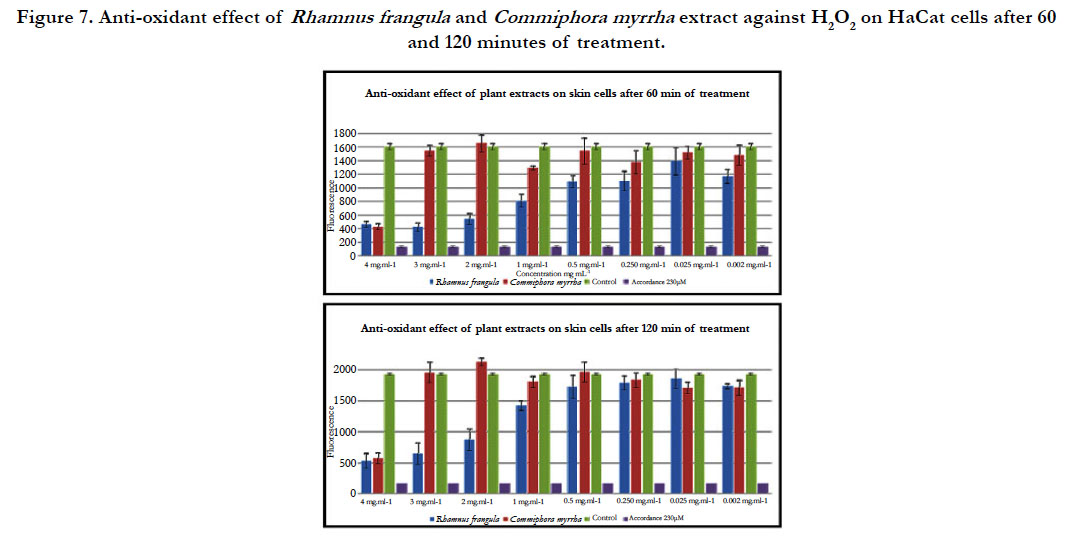
For the hot water extract of R. frangula ranging from 4 mg.mL-1 to 0.250 mg.mL–1, a strong reduction of ROS production by Ha- CaT cells was observed compared to the untreated sample. For the concentration ranging from 0.125 mg.mL-1 to 0.062 mg.mL–1 a little reduction of ROS production was observed compared to the untreated sample (figure 6). Indeed, after 60 minutes of treatment, a strong reduction in the production of ROS is observed for concentrations ranging from 4 to 0.250 mg .ml-1 (figure 7). However, after 120 minutes of treatment a sharp decline in the production of ROS is observed for concentrations ranging from 4 to 1 mg.mL-1 (figure 7). It could be that the antioxidant capacity of the R. frangula hot water extract has been consumed at the level of the low concentrations.
Figure 6. Anti-oxidant effect of Rhamnus frangula and Commiphora myrrha extract against H2O2 on HaCat cells.
For the hot water extract of C. myrrha, a strong reduction of ROS production was observed compared to the untreated sample only for the concentration at 4 mg.ml-1. For the other concentration (3 to 0.062 mg.mL-1), there is no reduction of ROS production by HaCaT cells (figure 6). Indeed, after 60 minutes and 120 minutes, a decrease in the production of ROS is observed only for the highest concentration (4 mg.mL-1) (figure 7).
Figure 7. Anti-oxidant effect of Rhamnus frangula and Commiphora myrrha extract against H2O2 on HaCat cells after 60 and 120 minutes of treatment.
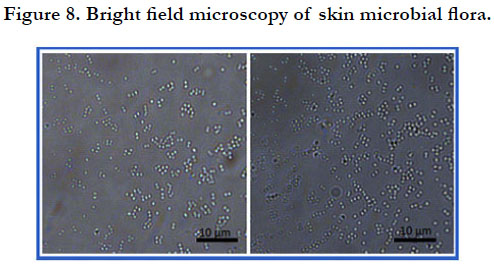
The bright field microscopy of skin microbial flora shows the
presence of bacteria in cluster or in diplococcic (figure 8). These
morphologies of bacteria suggest they belong to the genus Staphylococcus
and Streptococcus.
The microbial activity of the plant extracts of R. frangula and C. myrrha were studied in vitro with different concentrations (0.25, 0.5, 1, 2, and 3 mg/ml) against skin microbial flora.
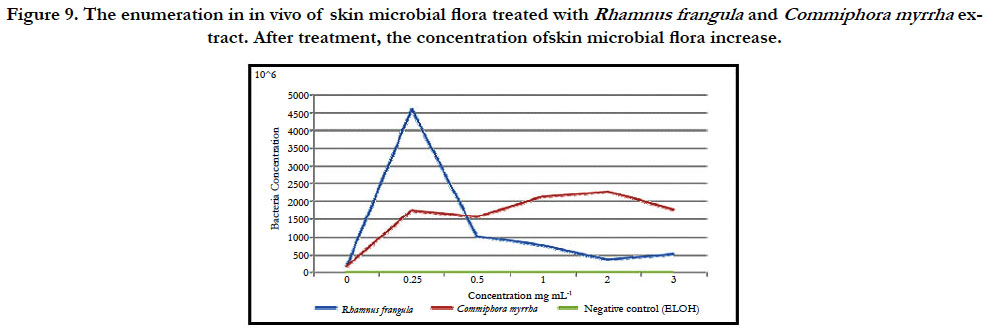
The enumeration of bacteria shows a strong increase in proliferation of skin microbial flora treated with 0.25 mg.ml-1 hot water extract of R. frangula compared to untreated samples (figure 9). For the concentration ranging from 0.5 mg.mL-1 to 3 mg.mL-1, the concentration of bacteria is equivalent to that of untreated samples.
The enumeration of bacteria shows an increase in skin microbial flora growth treated with 0.25 mg.ml-1 hot water extract of C. myrrha compared to untreated samples (figure 9). For the concentration ranging from 0.5 mg.mL-1 to 3 mg.mL–1, the concentration of bacteria is equivalent to that of the samples treated with 0.25 mg.ml-1 of hot water extract of C. myrrha. The treatment with 1 ml of ethanol is the negative control.
Figure 9. The enumeration in in vivo of skin microbial flora treated with Rhamnus frangula and Commiphora myrrha extract. After treatment, the concentration ofskin microbial flora increase.
In order to investigate whether hot plant extract activate defense
gene markers in HaCaT cells, we examined the expression changes
of some selected genes in response to extracts from R. frangula
and C. myrrha at 0.25 mg.ml-1 over a time course by real-time
quantitative reverse transcription (qRT)-PCR (figure 10). We have
chosen this concentration because it is a non-lethal concentration
to the cells.
Figure 10. Procedure to ectraction of RNA in HaCat cells after treatment with hot water extract of Rhamnus frangula and Commiphora myrrha at 1h, 3h, 6h, 12h and 48h compared at HaCat cells untreated.
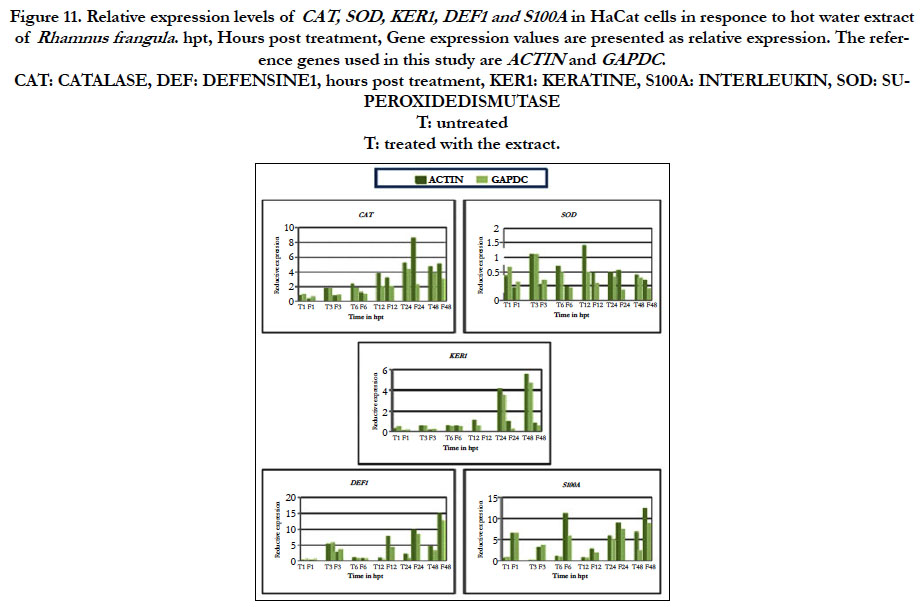
For this study we have selected five genes: CATALASE (CAT), SUPEROXIDE DISMUTASE (SOD), KERATIN1 (KER1), DEFENSIN1 (DEF1), INTERLEUKIN (S100A). The gene CAT encoding a catalase that is important for protecting the cells from oxidative damage caused by the ROS. After treatment with the extracts from both R. frangula and C. myrrha, the expression of CAT gene is not induced (figure 11 and 12). The SOD gene encodes for an enzyme that catalyses the dismutation of superoxide (O2−) into oxygen and hydrogen peroxide. Thus, they are an important antioxidant defense in nearly all cells exposed to oxygen. After treatment with hot plant extract of R. frangula, we observe a reduction in SOD transcript at 1, 3, 6, 12 h after elicitation (figure 11). After treatment with hot plant extract of C. myrrhaan increase in SOD transcript at 1 h after elicitation (figure 12). The KER1 gene is a differentiation factor. The protein encoded by this gene is a member of the keratin gene family. The type II cytokeratins consist of basic or neutral proteins that are arranged in pairs of heterotypic keratin chains coexpressed during differentiation of simple and stratified epithelial tissues [5, 8]. This gene is down regulated at 24, 48 h after treatment with hot plant extract of R. frangul and C. myrrha. An induction of DEFENSINE 1 gene, one of the most important genes known to encode for a peptide with a strong antimicrobial activity occurs. This human defensin is upregulated from 12, 24, 48 h after elicitation with hot plant extract of R. frangula treatment (figure 11) and is up-regulated from 1 h with hot plant extract of C. myrrha (figure 12). The gene S100A gene encoding an interleukin that participate in the regulation of immune responses, inflammatory reactions. After treatment with hot plant extract of R. frangula, we observed an up-regulation at 1, 3, 6, 12, 24, 48 h after treatment (figure 11). After treatment with hot plant extract of C. myrrha an increase in S100A transcript at 1 h after elicitation (figure 12).
Figure 11. Relative expression levels of CAT, SOD, KER1, DEF1 and S100A in HaCat cells in responce to hot water extract of Rhamnus frangula . hpt, Hours post treatment, Gene expression values are presented as relative expression. The reference genes used in this study are ACTIN and GAPDC.
Figure 12. Relative expression levels of CAT, SOD, KER1, DEF1 and S100A in HaCat cells in responce to hot water extract of C. myrrha . hpt, Hours post treatment, Gene expression values are presented as relative expression. The reference genes used in this study are ACTIN and GAPDC.
Discussion
The protective effects of extracts from both plants (R. frangula
and C. myrrha) endemic to Saudi Arabia were assessed on HaCaT
cells. ROS production is a well known marker of stress response.
In order to determine whether plant extracts are able to trigger an
immune response in HaCaT cells, ROS formation was monitored
using the fluorescent probe CM-H2DCFDA.
Extracts of both plants triggered ROS formation in HaCaT cells.
The hot water extracts from R. frangula and C. myrrha gererate ROS
in HaCaT cells within the first hour after treatment. However, the
intensity of the response was more important regarding the hot
water extract from R. frangula as compared to C. myrrha. These
findings reveal that extracts from these two saoudian plants are
able to stimulate an immune response in HaCaT cells.
The next step was to determine the range of concentration the
most appropriate for using these products as elicitors. In order
to determine if the plants extracts are cytotoxic to HaCaT cells,
cell viability and proliferation were determined by the MTT assay,
according to the method of Apone et al[1, 8]. For the R. frangula
extract, a loss of cell viability and proliferation occurred at concentrations
higher than 0.5 mg.mL-1. The C. myrrha extract reduces
the viability and proliferation at concentrations higher than 1
mg.mL-1. As a consequence, these products should not be used at
such higher concentrations. We consider that these extracts from
R. frangula and C. myrrha are not suitable for use as potential elicitors.
Indeed the concentration required to induce ROS is higher
than the toxicity value.
However, to rule out the possibility that the fluorescent probe
CM-H2DCFDA is not sufficiently sensitive in the cell system investigated,
expression of defense gene markers was investigated
in HaCaT cells in response to 0.25 mg.ml-1 treatment of extract
from R. frangula and C. myrrha. The expression of selected marker
genes was monitored in response to extracts from R. frangula and
C. myrrha at 0.25 mg.ml-1 over a time course by real-time quantitative
reverse transcription (qRT)-PCR. Surprisingly, the extracts
from both R. frangula and C. myrrha induce expression of defense
genes in HaCaT cell at such concentration. Moreover, the extract
from R. frangula induces the expression of more strongly defence
gene than extract from C. myrrha. These data confirmed that the
fluorescent probe CM-H2DCFDA was not sensitive enough to
detect ROS production in response to low concentration of plant
extracts.
As a consequence, based on (qRT)-PCR data, extracts from R. frangula and C. myrrha could be potential elicitors to trigger immune
response in HaCaT cells.
We investigated the potential property of these fractions to be
used as anti-oxidant molecules. To determine the anti-oxidant
capacity of the hot water extract of R. frangula and C.myrrha we
performed the two following in vitro tests: The Total Anti-oxidant
Capacity (TAC) and Oxygen Radical Absorbance Capacity
(ORAC). Additionally, an in vivo assay was performed. Our findings
reveal that the different plant extracts have a strong in vitro
anti-oxidant capacity. For the anti-oxidant effect of R. frangula and
C. myrrha H2O2 on HaCaT cells both plant extracts protect the
cell against the adverse effects of H2O2. Interestingly, our findings reveal that both plant extracts could be used as potential antioxidant
molecules against oxidative stress. It should be noted that
concentrations used are very low and below the limit value for
toxicity.
To evaluate the effect of the hot water extract of R. frangula and
C. myrrha skin microbial flora, we have performed one test in vitro.
The microbial activity of both plant extracts were studied in vitro
at different concentrations (0.25, 0.5, 1, 2, and 3 mg/ml) against
skin microbial flora. The enumeration of bacteria shows a strong
increase in skin microbial flora growth treated with 0.25 mg.ml-1
hot water extract of R. frangula compared to the non-treated flora.
The enumeration of bacteria shown an increase in skin microbial
flora growth treated with 0.25 mg.ml-1 hot water extract of C. myrrha compared to the skin microbial flora untreated. Thus, the
different plant extracts used here are not harmful to the natural
cutaneous microbial flora.
The results obtained with extracts from two endemic plants of
Saudi Arabia R. frangula and C. myrrha are highly promising with
regards to skin protection.
The limit value for toxicity of the extracts from R. frangula and C. myrrha is 0.5 mg.mL-1 and 1 mg.mL-1 respectively.
Both extracts have a significant anti-oxidant activity. Therefore
these extracts could be used as potential anti-oxidant molecules
against oxidative stress. It is worth noting that concentrations
used are below the limit value for toxicity.
The extracts have no negative effect for the natural cutaneous
microflora.
Acknowledgement
This research was funded by the Deanship of Scientific Research
at Princess Nourah bint Abdulrahman University through the
Fast-track Research Funding Program.
References
- Apone F, Tito A, Carola A, Arciello S, Tortora A, Filippini L, et al. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. Journal of Biotechnology. 2010; 145: 367–376. PMID: 20018216.
- Cui Y, Kim D-S, Park K-C. Antioxidant effect of Inonotus obliquus. Journal of Ethnopharmacology. 2005; 96: 79–85. PMID: 15588653.
- Kosalec I, Kremer D, Locatelli M, Epifano F, Genovese S, Carlucci G, et al. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chemistry. 2013; 136: 335–341. PMID: 23122067.
- Pastore S, Lulli D, Potapovich AI, Fidanza P, Kostyuk VA, Dellambra E, et al. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized Ha- CaT cells. Journal of Dermatological Science. 2011; 63: 104–114. PMID: 21620684.
- Seo SJ, Ahn SW, Hong CK, Ro BI. Expressions of β-defensins in human keratinocyte cell lines. Journal of Dermatological Science. 2001; 27: 183–191. PMID: 21620684.
- Stef D-S, Iosif G, Ioan-Trasca T, Stef L, Pop C, Harmanescu M, et al. Evaluation of 33 medicinal plant extracts for the antioxidant capacity and total phenols. Journal of Food, Agriculture & Environment. 2010; 8: 207–210.
- Thongrakard V, Tencomnao T. Modulatory effects of Thai medicinal plant extract on proinflammatory cytokines-induced apoptosis in human keratinocyte HaCaT cells. African Journal of Biotechnology. 2013; 9: 4999–5003.
- Walker D, Sun T, Macneil S, Smallwood R. Modeling the Effect of Exogenous Calcium on Keratinocyte and HaCaT Cell Proliferation and Differentiation Using an Agent-Based Computational Paradigm. Tissue Engineering. 2006; 12: 2301–2309. PMID: 16968170.