Recurrent Aphthous Stomatitis: A mini - Narrative Review
Melika Mokhtari*
Student Research Committee, Faculty Of Dentistry, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
*Corresponding Author
Melika Mokhtari,
Student Research Committee, Faculty Of Dentistry, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
Tel: +989120037207
Fax: +982122843352
E-mail: melikamokhtari777@gmail.com
Received: May 18, 2021; Accepted: November 13, 2021; Published: November 22, 2021
Citation: Melika Mokhtari. Recurrent Aphthous Stomatitis: A mini - Narrative Review. Int J Dentistry Oral Sci. 2021;8(11):5084-5090. doi: dx.doi.org/10.19070/2377-8075-210001023
Copyright: Melika Mokhtari©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Recurrent aphthous stomatitis (RAS) is a medical term with different meaning for practitioners unclear etiopathogenesis and no definite treatment ladder with lots of challenging issues in over-lap with other disease of oral cavity, for patient recurrent painful lesion in mouth which can im-pact functionally, psychosocially and economically .Diversity of causes and hypothesis each year lead to hundreds of researches to finding out new elements involving patients in this tragic disease but yet pin-pointing a precise etiological factor is difficult.RAS In terms of clinical pres-entation has 3 subtypes: minor (>70% of cases), major (10%), and herpetiform (10%).RAS leads to 25 percent of recurrent oral ulcers in adults and 40 percent in children.Lots of differential di-agnosis for recurrent aphthous ulcerations like idiopathic benign causes, inherited fever syn-dromes, connective tissue disease, gluten-sensitive enteropathy (celiac), ulcerative colitis and Crohn disease should be ruled out by comprehensive history taking and physical exam to find out whether it is related to a systemic inflammatory process or truly idiopathic. More investigation should be done to isolate the etiology of RAS for every case regarding the efficiency of con-ventional and unconventional treatment options, and patients tolerance of side effects.In this re-view of literatures, we trying to summarize results of different studies related to latest break-through and ethiological modalities for RAS to facilitate accurate diagnosis, proper classifica-tion, recognition of provocative factors, and the identification of associated diseases.
2.Introduction
3.Materials and Methods
3.Results
4.Discussion
5.Conclusion
5.References
Keywords
Aphthous Stomatitis; Oral Ulcer; RAS; Recurrent Aphthous Ulceration.
Introduction
Recurrent Aphthous Stomatitis (RAS) which has another name;
canker sores(the Greek word aphthi, which means “to set on
fire” or “to inflame) is one of thechronic, inflammatory diseases
of the oral cavity(oral mucosa of the lips, cheeks, keratinized
palatal,gingival mucosa and ton-gue)with painful oral ulcer without
preciseetiologyand with recurrence of one or more erosion or
ulcer each year, in the absenceof systemic abnormality or ulcer in
other parts of the body [1-4].The ulcers are painful and shallow
accompanied by grayish-white pseudomembrane plus an erythematous
margin [5]. Simple aphthosis, complex aphthosis, Recurrent
Oral Ulcers (ROU), and Recurrent Aphthous Ulcers (RAU)
should be regarded as synonyms for RAS [6]. In this review of
literature, we are trying to summarize the results of different studies
related to the latest breakthrough and etiological modalities
for Recurrent aphthous stomatitis (RAS) to facilitate precise diagnosis,
appropriate classification, identification of provocative
factors, and the recog-nition of related diseases.
Methods
This review was conducted using PubMed, SID, ISI Web of Science,
Scopus, Scholar in an at-tempt to find all articles relevant to
the recurrent aphthous stomatitis published until 2020. Ar-ticles
published in English were included. Studies that were similar or
duplicated were excluded. We chose the related studies based on
the title and abstract of the papers. We checked the refer-ences of
selected publications after obtaining the full text to see whether
there were any addi-tional studies.
Our search strategy in Pubmed was as follows:
(recurrent Aphthous Stomatitis [Title/Abstract]) OR (Aphthous Ulcer[Title/Abstract]) OR (Aph-thous Ulcers[Title/Abstract])
OR (Aphthae[Title/Abstract])) OR (Canker Sore[Title/Abstract])
OR (Canker Sores[Title/Abstract]) OR (Periadenitis Mucosa
NecroticaRecurrens[Title/Abstract])
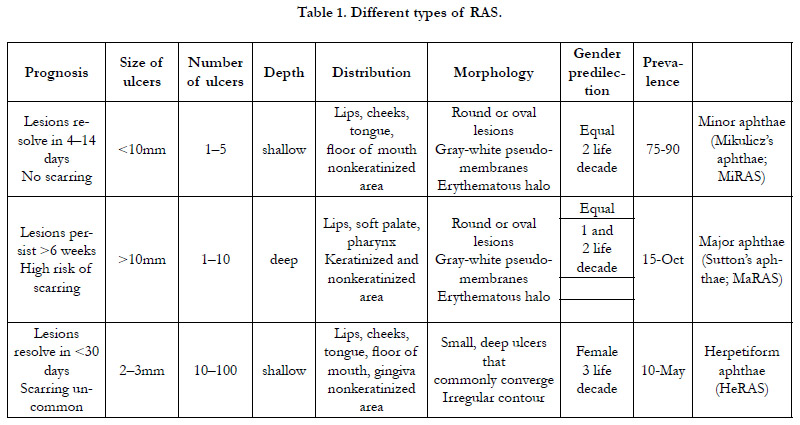
Categories of RAS
Based on morphology,RAS has three categories. After evaluation
of its clinical characteristic-spractitioners will decide which type
of RAS fired the situation: minor ulcers, major ulcers, or herpetiform
Ulcers [7, 8].
Minor RAS: the most common group 75–90% of patients, it is
less than 10mm in diameter and typically takes 4-14 days to heal
without scarring.Minor RAS appears on non-keratinized mucosa;
therefore, it is notcommon on dorsum of tongue, palate or gingiva
surfaces. It occurs more frequently in floor of mouth, buccal
mucosa, and labial mucosa. Ulcersare concentrated in the anterior
part of the mouth and are superficial.
Major RAS: ulcers are less common in comparison with Minor
RAS with 10-15 % of cases, deeper, often scarred, maybe with
limitation in tongue movements and speech. They exceed 10mm
and persist up for weeks.
Herpetiform ulceration: 5% of cases. A similarity between this
term and Herpes Simplex Virus (HSV) herpes labialis (fever blisters,
cold sores) infection exists but each one is a distinct entity.
5-100 ulcers with 2-3 mm in diameter may be present at the same
time. They are grey and have no delineated erythematous border,
therefore it is difficult to visualize them [7, 9, 10].
these groups features are vsummarized in Table 1.
Another method of categorizing RAS is using clinical features
of the disease:Mild type is called simple and Severe type
is called complex aphthosis [11, 12]. Most patientshave simple
ulcers but patients with a history of anemia, Inflammatory
Bowel Disease(IBD),celiac,Human Immunodefi-ciency Virus
(HIV), systemic lupus erythematous, cyclic neutropenia, Behcet’s
disease,or other such illnesses experience thecomplex type.
Simple aphthosis types are common, limited to oral cavity, andepisodic.
They cause few ulcers, minimal pain, 3-6 episodes per
year, little disability and have prompt healing.
Complex aphthosis types are uncommon, continuous or episodic.
They cause few to many ulc-ers,marked pain, frequent or continuous
ulceration, major disability and have slow healing and-may
cause genital aphthae.[12]
RAS is a worldwide known oral ulcer and a patient can be marked
with this title only by ruling out other oral ulcerative diseases secondary
to systemic diseases, and nutritional or haematologi-caldeficiencies.
The estimation of RAS prevalence in each country
varies because of the design of studies and number of patients
in previous surveys, qualification criteria and origin of the examined
subjects.
The prevalence ranges of RAS:
RAS onset is in childhood and the second decade of life considering
the peak period. The severity and recurrence tendency
to diminish with age may be due to alterations of the immune
system(decrease in the neutrophils’ chemotactic and phagocytic
capacity) [13, 14]. So in patients older than 40 it’s not common
[15]. Some literature’s mentioned that rich people, nonsmokers,
women, female dental and medical school students and student
nurses are more prone to expe-rience RAS and men are at lower
risk [16, 17]. RAS prevalence estimation has a wide range from
2% to 66% in different studies. Konopka in Poland in 2004 reported
RAS prevalence near 10 percent. In physical exam of 1281
patients [18]. However in patient history taking in 2009 by Safa-ri
in Jordan, RAS prevalence in 13 to 68-year-olds wasclaimed to be
near 78.1% [19]. RAS re-currence is about 3 to 6 times each year
and lesions which remain for 14 days are categorized as simple aphthosis but if these painful ulcers remain more than 14 daysand
emerge in other areas except mouth like genital mucosa,they arecategorized
as complex aphthosis [20-25]. If the ulcer of mouth
start in childhood rarely accompanies autoimmune diseasebut in
third decade, they can be more severe and sometimes the presentation
is Herpetiform RAS [26-28].
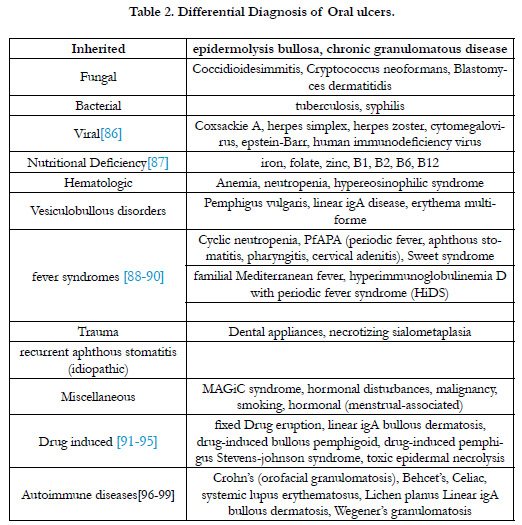
Oral Ulcerations’ Differential Diagnosis:
The first step in approaching a patient's complaint about mucosal
aphthous ulcer is listing the differential diagnosis and finding the
most matching disease with the patient's presentation. Al-though
RAS is common among individuals, lots of diseases have similarities
in signs and symp-toms with it. Therefore, before marking patients
with RAS, practitioners should rule out diseases which are
categorized in Table 2: infections (bacteria, virus,fungi) regularly
with vesiculobullous eruptions, trauma due to dental appliances,
Hormonal changes, malnutrition, gastrointestinal (GI) diseases,
autoimmune diseases with oral manifestations and drugs’ side effects.
Complete Physi-cal examination and comprehensive history
taking are two wings to facilitate diagnosing among differential
diagnosis [29] as mentioned in Table 2.
The mechanism of the development RAS:
Different studies have been done till now to find out predisposing
factors that could trigger the RAS in childhood and adolescence
and numerous items claimed to have a major or minor role in this
disease. However, the precise etiology is not clear overall. It is
assumed that the coincidence of particular trigger factors kicks
off the cascade of pro-inflammatory cytokines, directed against
sensitive oral mucosa areas, and aphthous will emerge. We should
be thankful to our colleagues in all medical research centers for
their efforts and here we summarize their findings hope that it
could facilitate future studies.
Viral and bacterial infections, stress, malnutrition, hematological
deficiencies, Hormonal level fluctuations, genetic predisposition,
drugs immune deficiency disorders, trauma and mechanical injuries,
and systemic diseases claimed to have role in this mysterious
disease [16, 24, 28, 30, 31].
Pathology study of oral RAS samples reported massive leukocytic
infiltration with dominancy oflymphocytes (mostly of the
T type) and monocytes inthe primary phase and in long-lasting
ulcers dominancy of polynuclear leukocytes was reported which
is similar to Behcet’s disease, lupus erythematous and other inflammatory
conditions and it isn’t an exclusive feature [32, 33].
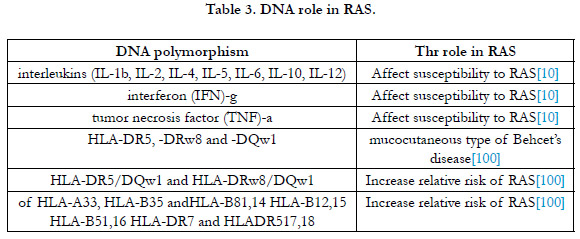
Inheritance:
Ship [34] first in 1965 reported the role of genetics in RAS in
families and other studies men-tioned that 24-46% of RAS patients
have a family history of recurrent aphthous in mouth [35].
Monozygotic vs dizygotic twins have more chance to be affected
by aphthous and due to past history their manifestations in recurrence
and severity are worse in comparison to those with no family
history..[36, 37] DNA polymorphism related to metabolism of
interleukins (IL-2, IL-5, IL-1b, IL-6, IL-12, IL-4, IL-10), tumor
necrosis factor (TNF) and interferon (IFN)-c, and Human Leukocyte
Antigen (HLA)-A33, HLA-B5, HLA-B12, HLA-B35, HLAB51,
HLA-B81, HLA-DR4, and HLA-DR7, have been proved in
RAS presentations [38-50].
Tobacco:
RAS in smokers has a lower rate due to the reduction of proinflammatory
cytokines (IL-6, IL-1 and TNF-a).Reactive oral mucosa
keratinization also causes a kind of protection against trauma
[27, 51].
Drugs:
It was reported that typical aphthous ulcers' clinical description
or/and clinical presentation indi-cating the aphthous ulcers' diagnosis
have been recorded for eight drugs [8, 52]: Sodium hypochlorite
[53], piroxicam [54], phenobarbital [55], phenindione [56]
niflumic acid [56], nicoran-dil [57], gold salts [56], captopril [58].
Infectious factors:
Bacteria like (Helicobacter pylori, Streptococcus oralis)and viral
(HIV-infected per-sons, cytomegalo virus, herpes simplex virus,
varicella-zoster virus, adenoviruses) antigens in dif-ferent studies
were proved to have prominent ties with RAS.In higher B12
serum level the RAS after eradicatin of H.pyloriRAS tend to be
reduced which served as a significant document that H.pylori role
in RAS should be a target point [28, 59-63].
Diet component role in RAS:
Food and microelement deficiencies such as vitamins and minerals
(vitamin B12, zinc, folic acid, microelement, iron) were proved
to be in relation to more prevalence of RAS and excessive exposure
to some food elements like date, raisin, gluten, preservatives,
food coloring agents, chocolate,cow milk, and nuts can lead
to RAS [5, 28, 64-69]. A meta-analysis by Al-Maweri et al.[70]
suggests that there is a significant relation between low levels of
vitamin D and RAS.
Systemic Diseases:
Celiac disease and chronic inflammatory bowel diseases (ulcerative
colitis, Crohn’s disease) by two pathophysiologies tend to present
RAS in the mouth; 1-food and microelement deficiencies,
2-autoimmune reactions [3, 28, 63, 71].
Hormonal Imbalance:
Serum levels of sex hormones in the luteal phase and Oral Contraceptive
Pills (OCPs) in case of contraceptive will lead to RAS
[28, 72].
Minor trauma:
A majority of patientswho suffered from RAS claim that lesions
often appear following trauma without disclosed reason till now
[28, 72, 73].
Stress:
Tention playsa key role in the occuranceof RAS by modifying immune
response system [28, 74-76].
Immunological mediated mechanisms in RAS:
It was proved by lots of recently studies that Immunologic response
disruption has footprints in RAS and drives the pathogenesis.
For better management of such disabling disease having
a comprehensive concept of immune response is necessary.
Increase in Pro-inflammatory cytokine-soriginated byT helper 1
(Th1)(IFN-c, IL-12, IL-2, TNF-a ),known as trigger factors of
autoim-munization by cellular type, and decrease of anti-inflammatory
cytokines with the source of Th2(IL-13, IL-5, IL-4 and
IL-10) and (TGF)-bwork by immunoglobulin E (IgE) in humoral
re-sponse system have been implicated as triggers of RAS [14,
42, 43, 77-82]. As lymphocyte im-migrate to themucosa of the
mouth, pro-inflammatory cytokines’secretion by activation of T
cells and TNF-a will affect some specific areas of buccal mucosa
ends to neutrophils migration to the lesions with ulcer manifestation
[83]. In patients with lower IL-10 level, RAS ulcers last more
than others due to its role inhealing process [40, 42, 71]. Higher
secretion of Th1 cytokines, neutrophils’ reactivation, increased
NK cells and B lymphocytes disproportion CD4/CD ratio lead
to RAS [80, 84, 85].
In some papers DNA polymorphism was proved to have different
effect on RAS as mentioned in Table 3.
Conclusion And Future Perspectives
This review was designed to explore the precise mechanisms of
RAS (which occurs as round, shallow, recurrent, oral ulcerations
surrounded by inflammation). Since it is the most common oral
mucosal lesion in the general population,therefore, it will be of
great interest to de-velop a list of specific factors influencing the
development of oral ulcers. The specific etiology of RAS remains
unclear despite extensive researches, but several systemic, genetic,
nutritional, microbial, local, immunologic, and allergic factors
have been suggested in different studies. Re-cent studies suggest that genetics plays a prominent part in combination with environmental
fac-tors in the development of RAS so we tried to prepare
a brief review of all factors which havea role in this disease.By a
deep evaluating of different studies, it will be disclosed that genetics
besides environmental modalities has a great impact on the
incidence and morbidities of RAS. Therefore, in future studies all
these factors must be regarded in a unique structure, not as different
isolated items, otherwise, no remedy could eradicate RAS.
References
-
[1]. Limbrock GJ, Fischer-Brandies H, Avalle C. Castillo-Morales' orofacial
therapy: treatment of 67 children with Down syndrome. Dev Med Child
Neurol. 1991 Apr;33(4):296-303. PubMed: 1828445.
[2]. Svensson H, Eriksson I. Oral motor therapy with palatal plates in children with Down syndrome.2017:38.
[3]. Marques LS, Alcântara CE, Pereira LJ, Ramos-Jorge ML. Down syndrome: a risk factor for malocclusion severity? Braz Oral Res. 2015;29:44. PubMed PMID: 25760064.
[4]. Limbrock GJ, Castillo-Morales R, Hoyer H, Stöver B, Onufer CN. The Castillo-Morales approach to orofacial pathology in Down syndrome. Int J Orofacial Myology. 1993 Nov;19:30-7. PubMed PMID: 9601231.
[5]. Alacam A, Kolcuoglu N. Effects of two types of appliances on orofacial dysfunctions of disabled children. The British Journal of Development Disabilities. 2007 Jul 1;53(105):111-23.
[6]. Bäckman B, Grevér-Sjölander AC, Bengtsson K, Persson J, Johansson I. Children with Down syndrome: oral development and morphology after use of palatal plates between 6 and 48 months of age. Int J Paediatr Dent. 2007 Jan;17(1):19-28. PubMed PMID: 17181575.
[7]. Macho V, Coelho A, Areias C, Macedo P, Andrade D. Craniofacial features and specific oral characteristics of Down syndrome children. Oral Health Dent Manag. 2014 Jun;13(2):408-11. PubMed PMID: 24984656.
[8]. Chad L. Critical Review: What are the effects of palatal plate therapy on orofacial features and speech in children with Down syndrome? In 2013.
[9]. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. PubMed PMID: 19621072.
[10]. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. [cited 2021 Apr 14]. Available from: /handbook/current
[11]. Risk of bias tools - ROBINS-I tool [Internet]. [cited 2021 Apr 14]. Available from: https://www.riskofbias.info/welcome/home
[12]. Carlstedt K, Henningsson G, McAllister A, Dahllöf G. Long-term effects of palatal plate therapy on oral motor function in children with Down syndrome evaluated by video registration. ActaOdontol Scand. 2001 Apr;59(2):63-8. PubMed PMID: 11370751.
[13]. Carlstedt K, Henningsson G, Dahllof G. A longitudinal study of palatal plate therapy in children with down syndrome. Effects on motor function. Journal of Disability and Oral Health. 2007;8(1):13.
[14]. Carlstedt K, Henningsson G, Dahllöf G. A four-year longitudinal study of palatal plate therapy in children with Down syndrome: effects on oral motor function, articulation and communication preferences. Acta Odontol Scand. 2003 Feb;61(1):39–46.
[15]. Matthews-Brzozowska T, Cudzilo D, Walasz J, Kawala B. Rehabilitation of the orofacial complex by means of a stimulating plate in children with Down syndrome. AdvClinExp Med. 2015 Mar-Apr;24(2):301-5. PubMed PMID: 25931364.
[16]. Bäckman B, Grevér-Sjölander AC, Holm AK, Johansson I. Children with Down Syndrome: oral development and morphology after use of palatal plates between 6 and 18 months of age. Int J Paediatr Dent. 2003 Sep;13(5):327-35. PubMed PMID: 12924988.
[17]. Matthews-Brzozowska T, Walasz J, Matthews-Kozanecka M, Matthews Z, Kopczynski P. The role of the orthodontist in the early simulating plate rehabilitation of children with Down syndrome.Journal of Medical Science. 2014 Jun 30;83(2):145-51.
[18]. Carlstedt K, Dahllöf G, Nilsson B, Modéer T. Effect of palatal plate therapy in children with Down syndrome. A 1-year study. Acta Odontol Scand. 1996 Apr;54(2):122-5. PubMed PMID: 8739145.
[19]. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021 Jan;12(1):55-61. PubMed PMID: 32336025.
[20]. Oliveira AC, Pordeus IA, Torres CS, Martins MT, Paiva SM. Feeding and nonnutritive sucking habits and prevalence of open bite and crossbite in children/ adolescents with Down syndrome. Angle Orthod. 2010 Jul;80(4):748- 53. PubMed PMID: 20482363.
[21]. Klingel D, Hohoff A, Kwiecien R, Wiechmann D, Stamm T. Growth of the hard palate in infants with Down syndrome compared with healthy infants- A retrospective case control study. PLoS One. 2017 Aug 10;12(8):e0182728. PubMed PMID: 28796822; PMCID: PMC5552113.
[22]. Korbmacher H, Limbrock J, Kahl-Nieke B. Orofacial development in children with Down's syndrome 12 years after early intervention with a stimulating plate. J OrofacOrthop. 2004 Jan;65(1):60-73. English, German. Pub- Med PMID: 14749890.
[23]. Rao D, Hegde S, Naik S, Shetty P. Malocclusion in Down syndrome - a review. 70(1):4.
[24]. Korbmacher HM, Limbrock JG, Kahl-Nieke B. Long-term evaluation of orofacial function in children with Down syndrome after treatment with a stimulating plate according to Castillo Morales. J ClinPediatr Dent. 2006 Summer;30(4):325-8. PubMed PMID: 16937860.
[25]. Hoyer H, Limbrock GJ. Orofacial regulation therapy in children with Down syndrome, using the methods and appliances of Castillo-Morales.ASDC J Dent Child. 1990 Nov-Dec;57(6):442-4. PubMed PMID: 2147926.