Clinical, Biological and Therapeutic Features of a Patient with Acro Dermato Ungual Lacrimal Tooth Syndrome
Marwa Trifi W1*, Bekri S2, Mansour L3, Trabelsi M3
1 DDS, Resident Fellow in Removable Partial Dentures, Faculty of Dental Medicine, University of Monastir, Tunisia.
2 DDS, Assistant Professor in Removable Partial Dentures, Faculty of Dental Medicine, University of Monastir, Tunisia.
3 DDS, Professor in Removable Partial Dentures, Faculty of Dental Medicine, University of Monastir, Tunisia.
*Corresponding Author
Wijdene Marwa Trifi DDS,
Resident Fellow in Removable Partial Dentures, Laboratory of Dento-Facial,
Clinical and Biological Approach (ABCDF), LR12ES10, Faculty of Dental Medicine,
University of Monastir, Avicenna Avenue, 5000, Monastir, Tunisia.
Tel/Fax: +21620562733
E-mail: trifiwijdene@yahoo.fr
Received: February 03, 2018; Accepted: February 22, 2018; Published: February 28, 2018
Citation:Marwa Trifi W, Bekri S, Mansour L, Trabelsi M. Clinical, Biological and Therapeutic Features of a Patient with Acro Dermato Ungual Lacrimal Tooth syndrome. Int J Dentistry Oral Sci. 2018;5(2):606-610. doi: dx.doi.org/10.19070/2377-8075-18000118
Copyright: Marwa Trifi W©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Acro-Dermato-Ungual-Lacrimal-Tooth syndrome is a rare autosomal dominant disorder resulting from TP63 gene mutation. ADULT syndrome is clinically manifested by ectodermal dysplasia, limb defects, nasolacrimal duct obstruction, absence of orofacial clefting, primary hypodontia and the early loss of permanent teeth. The aim of this report was to study the clinical, histological and therapeutic features of the early loss of permanent teeth of a 22-year-old Tunisian female.
Case Presentation: A 22-year-old female, born to clinically healthy and consanguineous parents, was referred to the removable partial denture department for full mouth rehabilitation. The patient’s medical history included a continuous follow up of her chronic dacryocystitis and a hand surgery at the age of 17. On general examination, the patient was mildly mentally retarded, underweight and photosensitive. She presented a fine hair, hypoplastic mammary glands and bilateral nasolacrimal duct obstruction complicated with corneal abscess. The examination of her digits showed onychodysplasia, fingers camptodactyly and toes syndactyly. The oral examination showed the presence of a temporary tooth and the early loss of several permanent teeth. The present teeth were peg shaped and mostly decayed. Enamel was poorly formed. Temporary and decayed permanent teeth were extracted. Polarized light microscopy was then performed to observe half teeth sections stained with hematoxylin-eosin. The other half was observed under scanning electron microscopy.
Results: The patient underwent full mouth rehabilitation with a complete fixed maxillary prosthesis and a combined mandibular prosthesis with extracoronal castable precision attachment. The histological findings revealed that enamel was hypoplastic, hypocalcified and dystrophic. The acellular extrinsic fiber cementum and vertical periodontal fibers were absent. The cellular intrinsic fiber cementum was present on the root layer and on a great part of the enamel surface.
Conclusion: The premature loss of permanent teeth reported in ADULT syndrome patients can be the consequent result of the absence the periodontal attachment.
2.Introduction
3.Materials and Methods
4.Results
4.1 Full Mouth Rehabilitation
4.2 Minor Salivary Glands
4.3 Mucosa
4.4 Enamel
4.5 Dentin
4.6 Cementum
5.Discussion
5.1 Mucosa.
5.2 Enamel.
5.3 Cementum.
6.Conclusion
7.References
Keywords
Ectodermal Dysplasia; ADULT Syndrome; Dental Cementum; Epithelium.
Introduction
Acro-Dermato-Ungual-Lacrimal-Tooth (ADULT) syndrome, also known as Propping Zerres syndrome and pigment anomalyectrodactyly- hypodontia (OMIM 103285, ORPHA 978), is a very rare ectodermal dysplasia. This syndrome was first described in 1993 by Propping and Zerres [1-3]. The prevalence is 1 in 1000000 and it is inherited autosomal-dominantly [4]. Heterozygous mutations in P63 gene have been identified as the cause [5]. P63, as a transcription factor, is crucial during embryonic ontogenesis, mostly in the development of limbs and other ectodermal derived tissues [6].
Symptoms include prominent ectodermal signs comprising syndactyly, ectrodactyly and ectodermal abnormalities such as finger and toenails dysplasia, intensive freckling and hypoplastic breasts and nipples [7]. The orofacial features, uncommonly studied, aresparse hair, lacrimal duct atresia [8], absence of lip and/or palate cleft, xerostomia, primary hypodontia and loss of permanent teeth because of weak fixation [9].
This paper reports a patient presenting the clinical features of ADULT syndrome and expands the spectrum of histological features of deciduous and permanent teeth.
Materials and Methods
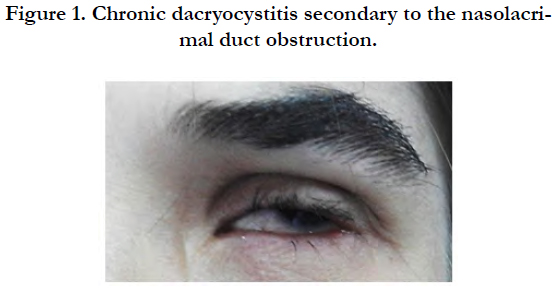
A 22-year-female, born to clinically healthy and consanguineous parents, was referred to the removable partial dentures department of the Dental Clinic of Monastir for full mouth rehabilitation. The patient’s medical history included a continuous follow up of her chronic dacryocystitis [Figure 1] since the age of 6 and a hand surgery at the age of 17.
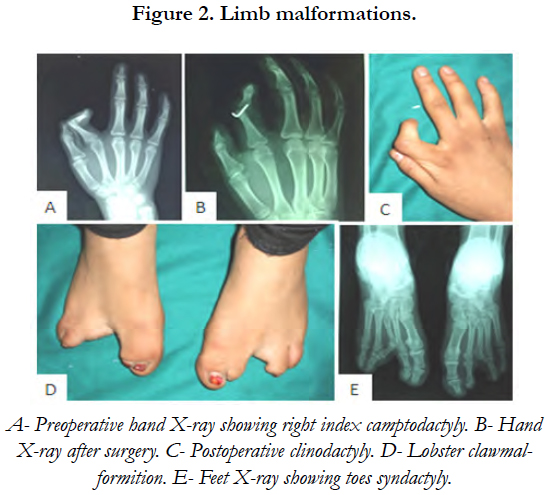
On general examination, the patient was mildly mentally retarded and underweight. Her hair was fine and since childhood, she has been suffering from photosensitivity. She presented bilateral nasolacrimal duct obstruction complicated with corneal abscess. This defect had been treated surgically by probing the duct obstruction to relieve the chronic dacryocystitis. The examination of her digits showed horizontal groves along the length of the nails, finger camptodactyly, toe syndactyly and ectrodactyly [Figure 2]. Her mammary glands were hypoplastic and her salivary glands were non palpable.
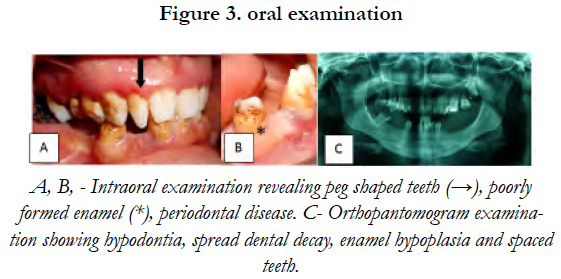
Oral examination showed peg shaped teeth, a poorly formed enamel, an abnormal cusp pattern and a considerable amount of dental decay [Figure 3].
Minor labial salivary glands biopsies were performed by midline incision of the lower lip under local anesthesia. Five minor salivary glands were harvested because of prominent variation in the degree of the inflammatory involvement of salivary glands and lobes. The biopsy was followed by histological examination after hematoxylin-eosin staining in order to evaluate the degree of lesion (frequency and intensity of ductal lesions fiber) using the CHOMETTE and coll classification.
Three teeth (1 temporary + 2 permanent) and mucosa were taken under local anesthesia. After extraction, teeth were fixed with para-formaldehyde for 10 days and sectioned in two parts. They were then dehydrated in a graded series of alcohol. Three sections were coated in a thin layer of gold (100 A° of thickness). The other three sections were stained in hematoxylin-eosin. Polarized light microscopy and scanning electron microscopy were then performed.
The treatment included the use of set up diagnostic wax-up and the diagnostic occlusal adjustment in order to establish the accurate diagnosis and the treatment planning.
The management has associated fixed prosthesis and precision attachments-retained removable partial prosthesis in order to reincrease the occlusal vertical dimension, restore the morphology of the residual teeth and replace those absent.
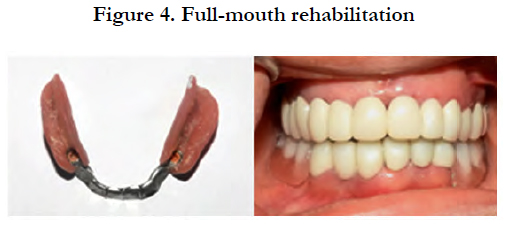
After several months of follow-up, the patient has not presented any symptoms and the rehabilitation appeared to be effective in balancing the functional stability and the cosmetic appeal [Figure 4].
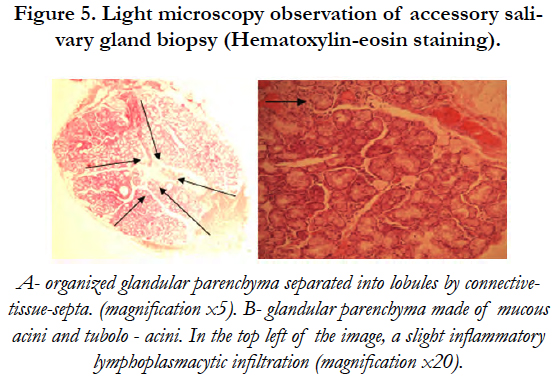
Polarized light microscopy (PLM) showed an organized glandular parenchyma made of mucous acini and tubuloacinar separated into lobules by connective-tissue-septa. A slight inflammatory infiltration of lymphocytes and plasma cells was also noticed [Figure 5].
Figure 5. Light microscopy observation of accessory salivary gland biopsy (Hematoxylin-eosin staining).
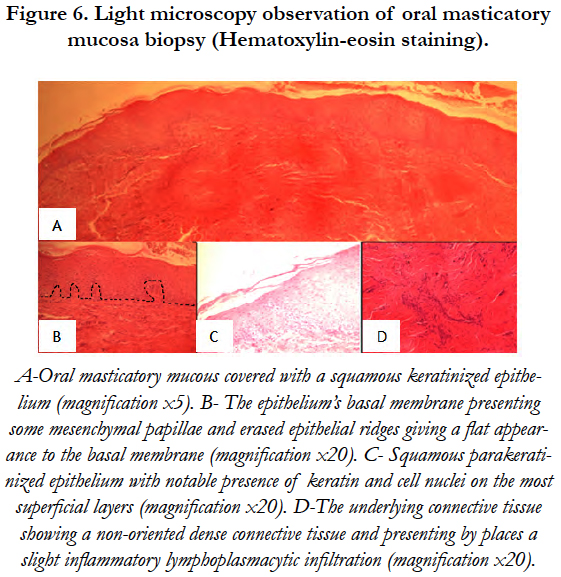
Under PLM, the observed mucosa was made of a squamous parakeratinized epithelium with notable presence of keratin and cell nuclei on the most superficial layers. The epithelium’s basal membrane presented some mesenchymal papillae and erased epithelial ridges which gave a flat appearance to the basal membrane. The underlying connective tissue showed a non oriented dense connective tissue, presenting by places a slight inflammatory lymphoplasmacytic infiltration [Figure 6].
Figure 6. Light microscopy observation of oral masticatory mucosa biopsy (Hematoxylin-eosin staining).
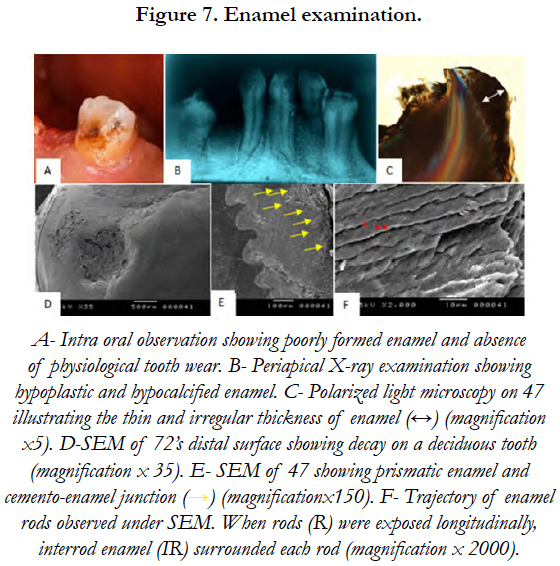
PLM revealed the presence of dystrophic and globular enamel. The scanning electron microscopy of tooth’s (72) distal surface showed not only the absence of perikymata on enamel outlayer but also a cellular cementum layer covering a great part of enamel surface. SEM performed on the impacted lower second molar (47) showed normal rods trajectory in the inner two thirds of the enamel layer and visualized the rod-interrod relationship [Figure 7].
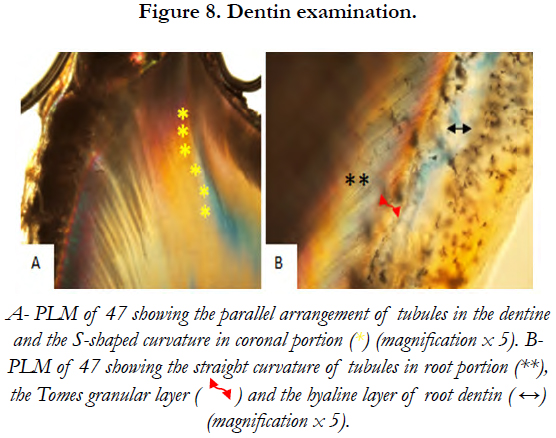
Dentinal tubular appearance and the parallel arrangement of tubules in dentine below the enamel were observed on PLM. These tubules showed an S-shaped curvature in the coronal portion and a more straight curvature in the root portion [Figure 8].
PLM revealed the presence of a thick cellular cementum layer on tooth (47). Indeed, a big amount of cellular cementum was present near the crown. It was dense in cementocytes and some empty cementoblasts were found. The enamel outer layer covered with cellular intrinsic fiber cementum (CIFC) was also observed on the SEM.
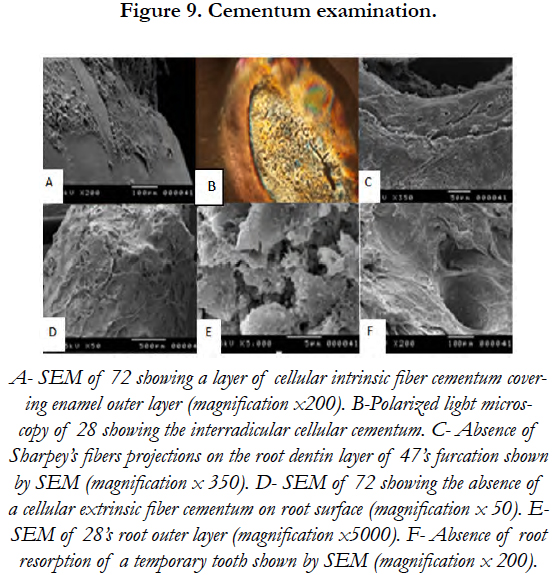
As for root, the outer layer of the deciduous and permanent teeth was also covered by CIFC. A cellular extrinsic fiber cementum (AEFC) was not noticed. SEM revealed the absence of Sharpey’s fibers projections on the root dentin layer of tooth (47)’s furcation [Figure 9].
Discussion
Decay [10-13], xerostomia [4, 8, 14], hypodontia [1, 4, 7-9, 14-19] and premature loss of permanent teeth are commonly reported features in ADULT syndrome. However, these oral features have been largely unexplored within the scientific community.
Thurfjell [20] described the function of P63 in mucosa development. He reported that knockout mice, in which the P63 gene has been functionally deleted, had shown severe developmental abnormalities of squamous epithelia and die rapidly due to dehydration, Kock [21] stated that P63 has been also observed in the nuclei and epithelial cells of stratified epithelia in human tissues. These data indicate that P63 expression is vital for the development of oral mucosa.
Enamel defects may result from P63 mutation affecting the enamel embryonic development. Indeed, according to Kock’s [21] study of P63 expression in human prenatal tooth primordia, there was a marked positive reaction to P63 in both cap stage and bell stage. In the cap stage, a strong positivity was noticed in the inner and outer enamel epithelium, in dental lamina and in the enamel knot [8]. Thesleff [22] reported that P63 mutations affect Wnt and BMP signals regulating the development of enamel knot and affects the dominant function of the enamel knot in the morphogenesis of the tooth crown [23]. Shh signal, which is essential for the epithelium proliferation and the regulation of the reciprocal interaction between the epithelium and the mesenchyme. is also affected by P63 mutations. Thus, the epithelium-derived enamel can be affected by P63 mutations and the deposition of this defective enamel, together with xerostomia, can be the susceptible reasons of caries [24]. In addition, P63 expression has a close link with the apoptosis of the enamel knot which arrests tooth development at the cap stage [21]. These findings explain, probably, the multiple agenesis of teeth seen in individuals with mutations in P63.
A cellular extrinsic fibers cementum (AEFC) is the tissue covering the cervical portion of the tooth root, important for attachment of the periodontal ligament (PDL) to the root surface [25]. In this study, the AEFC was absent on root surface and furcation. The consequent absence of Sharpey’s fibers projections on the root dentin layer revealed induced the absence of periodontal attachment. These results may explain the premature loss of deciduous and permanent teeth.
P63 expressed in the inner and outer enamel epithelium can interfere with the development of the adamantine epithelium and thus with the genesis of the the Hertwig’s epithelial enamel root sheath [26]. The cellular intrinsic fiber cementum (CIFC), covering a great part of enamel surface and the whole outer layer of the deciduous and permanent teeth in this study, may have an epithelium origin which concurs with Zeichner findings [27].
Conclusion
To our knowledge, this is the first report discussing the histological and the therapeutic features of the premature loss of permanent teeth described in ADULT syndrome patients.
Immunohistochemistry, osteoimmunology and genetic studies are proposed to clarify the developmental biology of tooth tissues in the case of ADULT syndrome.
References
- Propping P, Zerres K. ADULT‐syndrome: An autosomal‐dominant disorder with pigment anomalies, ectrodactyly, nail dysplasia, and hypodontia. Am J Med Genet. 1993 Mar 1;45(5):642-8. PubMed PMID: 8456838.
- Propping P, Friedl W, Wienker TF, Uhlhaas S, Zerres K. ADULT syndrome allelic to limb mammary syndrome (LMS)?. Am J Med Genet. 2000 Jan 17;90(2):179-82. PubMed PMID: 10607963.
- Amiel J, Bougeard G, Francannet C, Raclin V, Munnich A, Lyonnet S, Frebourg T. TP63 gene mutation in ADULT syndrome. Eur J Hum Genet. 2001 Aug;9(8):642-5. PubMed PMID: 11528512.
- Berk DR, Armstrong NL, Shinawi M, Whelan AJ. ADULT syndrome due to an R243W mutation in TP63. Int J Dermatol. 2012 Jun;51(6):693-6. doi: 10.1111/j.1365-4632.2011.05375.x. PubMed PMID: 22607287.
- Almeida HL, Caspary P, Duquia RP, Meijer R, van Steensel M. Adermatoglyphia, previously unrecognized manifestation in ADULT syndrome. Am J Med Genet A. 2010 Oct;152A(10):2656-7. doi: 10.1002/ajmg.a.33625. PubMed PMID: 20814947.
- Van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, et al. p63 Gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand–split foot malformation suggest a genotypephenotype correlation. Am J Hum Genet. 2001 Sep;69(3):481-92. Epub 2001 Jul 17. PubMed PMID: 11462173.
- Rinne T, Spadoni E, Kjaer KW, Danesino C, Larizza D, Kock M, et al. Delineation of the ADULT syndrome phenotype due to arginine 298 mutations of the p63 gene. Eur J Hum Genet. 2006 Aug;14(8):904-10. Epub 2006 May 17. PubMed PMID: 16724007.
- Eter N, Zerres K, Propping P, Roggenkämper P, Spitznas M. Severe persistent nasolacrimal duct obstruction: a typical finding in ADULT syndrome. Br J Ophthalmol. 2006 Sep;90(9):1206-7. PubMed PMID: 16929068.
- O'Brien KE, Shorrock J, Bianchi DW. Prenatal Diagnosis of Acro‐Dermato‐ Ungual‐Lacrimal‐Tooth Syndrome, a Dominantly Inherited Ectrodactyly. J Ultrasound Med. 2002 Aug;21(8):921-5. PubMed PMID: 12164578.
- Bedeschi MF, Escande F, Bellini M, Natacci F, Cavallari U, Lalatta F. Acrodermato- ungual-lacrimal-tooth-like syndrome: report of a family with variable expression. Clin Dysmorphol. 2006 Oct;15(4):239-41. PubMed PMID: 16957482.
- Reisler TT, Patton MA, Meagher PP. Further phenotypic and genetic variation in ADULT syndrome. Am J Med Genet A. 2006 Nov 15;140(22):2495- 500. PubMed PMID: 17041931.
- Ginige N, De Silva K, Senevirathne J. A case of acro-dermato-ungual-lacrimal- tooth syndrome with chronic parotitis: A new association?. Indian Journal of Paediatric Dermatology. 2014 Sep 1;15(3).
- Mohammad A. Unusual manifestations of ectodermal dysplasia-syndactyly syndrome type I in two Yemeni siblings. Dermatol Online J. 2015 Jan 15;21(1). pii: 13030/qt7cz9v3m0. PubMed PMID: 25612123.
- Avitan‐Hersh E, Indelman M, Bergman R, Sprecher E. ADULT syndrome caused by a mutation previously associated with EEC syndrome. Pediatr Dermatol. 2010 Nov-Dec;27(6):643-5. doi: 10.1111/j.1525-1470.2010.01131.x. Epub 2010 Nov 16. PubMed PMID: 21078104.
- Duijf PH, Vanmolkot KR, Propping P, Friedl W, et al. Gain-of-function mutation in ADULT syndrome reveals the presence of a second transactivation domain in p63. Hum Mol Genet. 2002 Apr 1;11(7):799-804. PubMed PMID: 11929852.
- Chan I, Harper JI, Mellerio JE, McGrath JA. ADULT ectodermal dysplasia syndrome resulting from the missense mutation R298Q in the p63 gene. Clin Exp Dermatol. 2004 Nov;29(6):669-72. PubMed PMID: 15550149.
- Slavotinek AM, Tanaka J, Winder A, Vargervik K, Haggstrom A, Bamshad M. Acro‐dermato‐ungual‐lacrimal‐tooth (ADULT) syndrome: report of a child with phenotypic overlap with ulnar‐mammary syndrome and a new mutation in TP63. Am J Med Genet A. 2005 Oct 1;138A(2):146-9. PubMed PMID: 16114047.
- Kier‐Swiatecka E, Kock M, Marker P, Eiberg H, Kjaer KW. The ADULTEEC spectrum: An R280C mutation with a borderline phenotype. Am J Med Genet A. 2007 Apr 15;143A(8):891-4. PubMed PMID: 17352394.
- Zelst‐Stams WA, Steensel MA. A novel TP63 mutation in family with ADULT syndrome presenting with eczema and hypothelia. Am J Med Genet A. 2009 Jul;149A(7):1558-60. doi: 10.1002/ajmg.a.32881. PubMed PMID: 19530185.
- Thurfjell N, Coates PJ, Boldrup L, Lindgren B, Bäcklund B, Uusitalo T, et al. Function and importance of p63 in normal oral mucosa and squamous cell carcinoma of the head and neck. Adv Otorhinolaryngol. 2005;62:49-57. PubMed PMID: 15608417.
- Kock M, Nolting D, Kjaer KW, Hansen BF, Kjær I. Immunohistochemical expression of p63 in human prenatal tooth primordia. Acta Odontol Scand. 2005 Oct;63(5):253-7. PubMed PMID: 16419429.
- Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006 Dec 1;140(23):2530-5. PubMed PMID: 16838332.
- Vaahtokari A, Åberg T, Jernvall J, Keränen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996 Jan;54(1):39-43. PubMed PMID: 8808404.
- Rinne T, Hamel B, Bokhoven HV, Brunner HG. Pattern of p63 mutations and their phenotypes-update. Am J Med Genet A. 2006 Jul 1;140(13):1396- 406. PubMed PMID: 16691622.
- Foster BL, Soenjaya Y, Nociti Jr FH, Holm E, et al. Deficiency in acellular cementum and periodontal attachment in bsp null mice. J Dent Res. 2013 Feb;92(2):166-72. doi: 10.1177/0022034512469026. Epub 2012 Nov 26. PubMed PMID: 23183644.
- Luan X, Ito Y, Diekwisch TG. Evolution and development of Hertwig's epithelial root sheath. Dev Dyn. 2006 May;235(5):1167-80. PubMed PMID: 16450392.
- Zeichner‐David M. Regeneration of periodontal tissues: cementogenesis revisited. Periodontol 2000. 2006;41:196-217. PubMed PMID: 16686935.